Gibbs Free Energy Calculator Electrochemistry
Students will be able to calculate Gibbs Free EnergyHow to use this video for Blended or Flipped Instruction. The change in Gibbs free energy for an electrochemical cell can be related to the cell potential.

Standard Cell Potential And Free Energy Worked Example Youtube
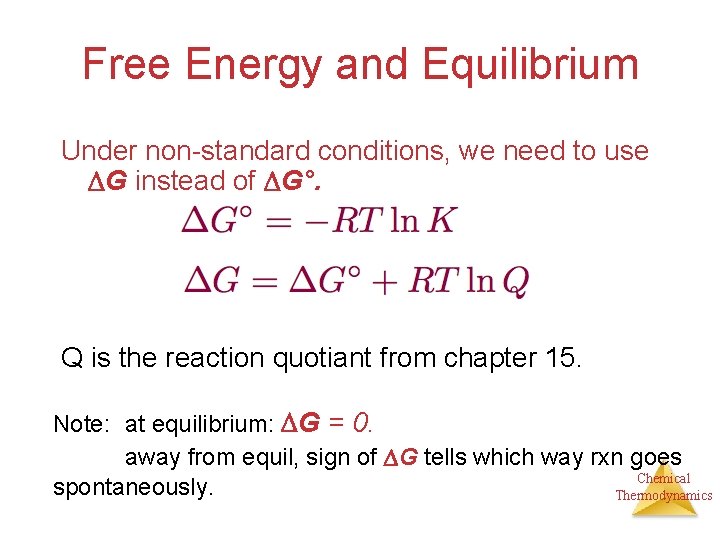
Two important important parameters that can be determined from a cell potential are the equilibrium constant for the cell reaction and the free energy change for the cell reaction.

Gibbs free energy calculator electrochemistry. For example in 139 we are asked to calculate the Gibbs Free Energy for 2Ce 4 aq 3I-aq-----2Ce 3 aq I 3-aq with an E cell of 108v. To use this online calculator for Electrode Potential when gibbs free energy is given enter Gibbs Free Energy Change G and Number of moles of electron n and hit the calculate button. Relationship between Gibbs free energy and standard cell potential.
Lets derive Nernst Equation. Equilibrium Constant and Free Energy Change for an Electrochemical Cell. Read free for 30 days.
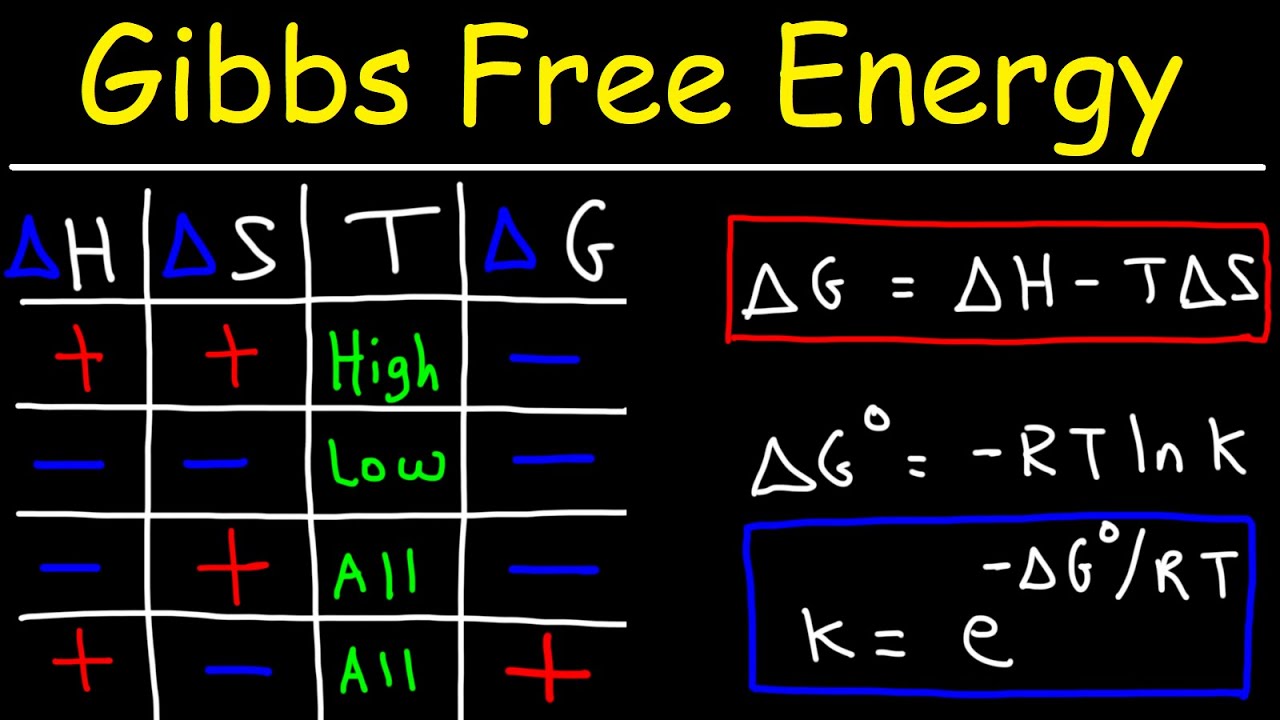
Here is how the Electrode Potential when gibbs free energy is given calculation can be explained with given input values - -1036E-5 -5050Faraday. Willard Gibbs defined a function known as Gibbs energy G to calculate the changes in entropy and enthalpy values. Measured in Joule per Kelvin Temperature - Temperature is the degree or intensity of heat present in a substance or object.
Electrode Potential when gibbs free energy is given Equilibrium Constant when degree of dissociation is given Excess pressure if the osmotic coefficient if given. Electrochemical cells convert chemical energy to electrical energy and vice versa. Calculate the standard Gibbs energy of the cell reaction Given.
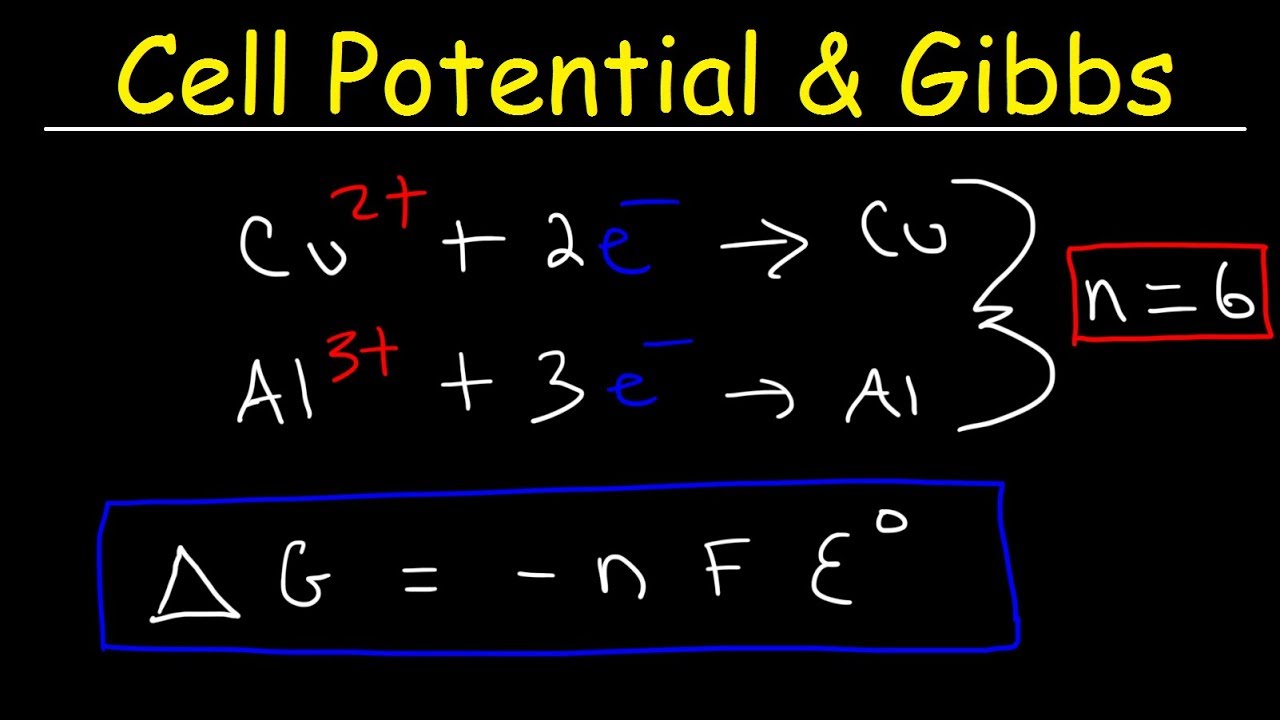
I understand that the n in G -nFE is the moles of the reaction but I dont understand how it is distinguished. Measured in Joule per Kelvin Gibbs free entropy - The Gibbs free entropy is an entropic thermodynamic potential analogous to the free energy. The Gibbs free energy of the system is a state function because it is defined in terms of.
It contains plenty of examples and chemistry practice. Gibbs Free energy formula is given below. The Change in Gibbs free energy if electrochemical work is given formula is defined as a decrease in free energy of collection provides a measure of electrochemical work dine by the cell is calculated using gibbs_free_energy -Work DoneTo calculate Change in Gibbs free energy if electrochemical work is given you need Work Done wWith our tool you need to enter the respective value for.
To use this online calculator for Cell potential if change in Gibbs free energy is given enter Gibbs Free Energy G and Moles of electron transferred n and hit the calculate button. This chemistry video tutorial discusses the relationship between cell potential and gibbs free energy. Electrochemistry Nernst Equation.
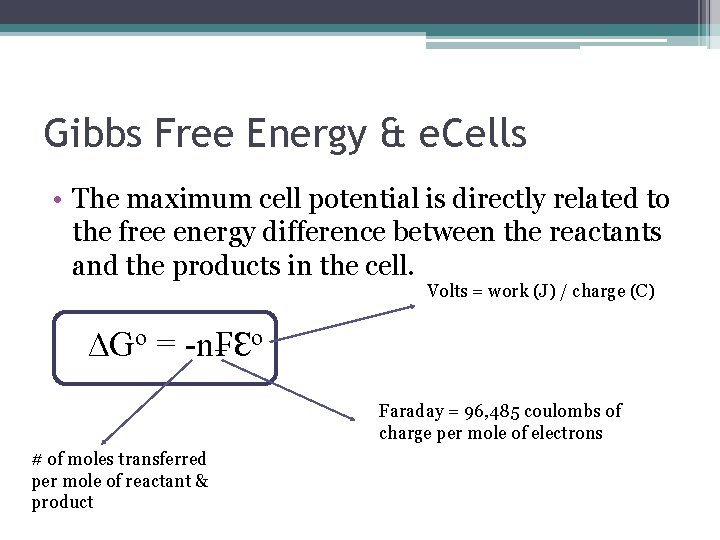
NFE nFEo RTln Q nF E nF E o RT ln Q. Delta G -nFE which is the equation used to determine the free energy change that accompanies a redox reaction in a voltaic celln moles of electrons tra. The maximum work done is the amount of energy produced given by the decrease in the thermodynamic property called Gibbs free energy.
The Relationship between Cell Potential Gibbs Energy. Chapter Two - View presentation slides online. Here n is the number of moles of electrons F is the Faraday constant Coulombs mole Coulombs mole and E is the cell potential.
Entropy - Entropy is the measure of a systems thermal energy per unit temperature that is unavailable for doing useful work. G H - TS. I dont see how the moles of this reaction is 2 when there are 3 moles of I-.
Using Playposit to add ques. Cell potential if electrochemical work is given. H change in enthalpy.
The total amount of energy produced by an electrochemical cell and thus the amount of energy available to do electrical work depends on both the cell potential and the total number of electrons that are transferred from the reductant to the oxidant during. Watch the next lesson. 1F 96500 Cmol CBSE 2017 The standard Gibbs free energy G can be obtained from the equation.
Here is how the Cell potential if change in Gibbs free energy is given calculation can be explained with given input values - -0000592 -228614Faraday. Nernst Equation is used to calculate EMF of a cell. Actual mass if current efficiency is given.
Therefore Gibbs theory is. The Gibbs free energy of a system at any moment in time is defined as the enthalpy of the system minus the product of the temperature times the entropy of the system. At temperature T change in Gibbs Free Energy is given by.
G G0 RTlnQ where Q Reaction quotient R Universal Gas Constant. Has E o cell 0236 V at 298 K.
Cell Potential Gibbs Free Energy Standard Reduction Potentials Electrochemistry Problems Youtube

Cell Voltage And Gibbs Free Energy Youtube

Thermodynamic Cycle For The Calculation Of Deprotonation Gibbs Free Download Scientific Diagram

Free Energy And Redox Reactions Ppt Download
Free Energy G Nernst Equation 20 5 20

1 The Laws Of Thermodynamics In Review 1 The Internal Energy Of An Isolated System Is Constant There Are Only Two Ways To Change Internal Energy Heat Ppt Download

How To Calculate Change In Gibb S Free Energy Of Reaction Using Gibbs Formation Example Problems Youtube
Chemistry The Central Science 10 Th Edition Theodore

High Temperature Muteriulr And Aonuses Standard Gibbs Free Energies Of Download Table

Gibbs Free Energy Example Shefalitayal

Gibbs Free Energy And Spontaneity Article Khan Academy
Gibbs Free Energy Entropy Enthalpy Equilibrium Constant K Youtube

Gibbs Free Energy And Calculations Example 3 Youtube
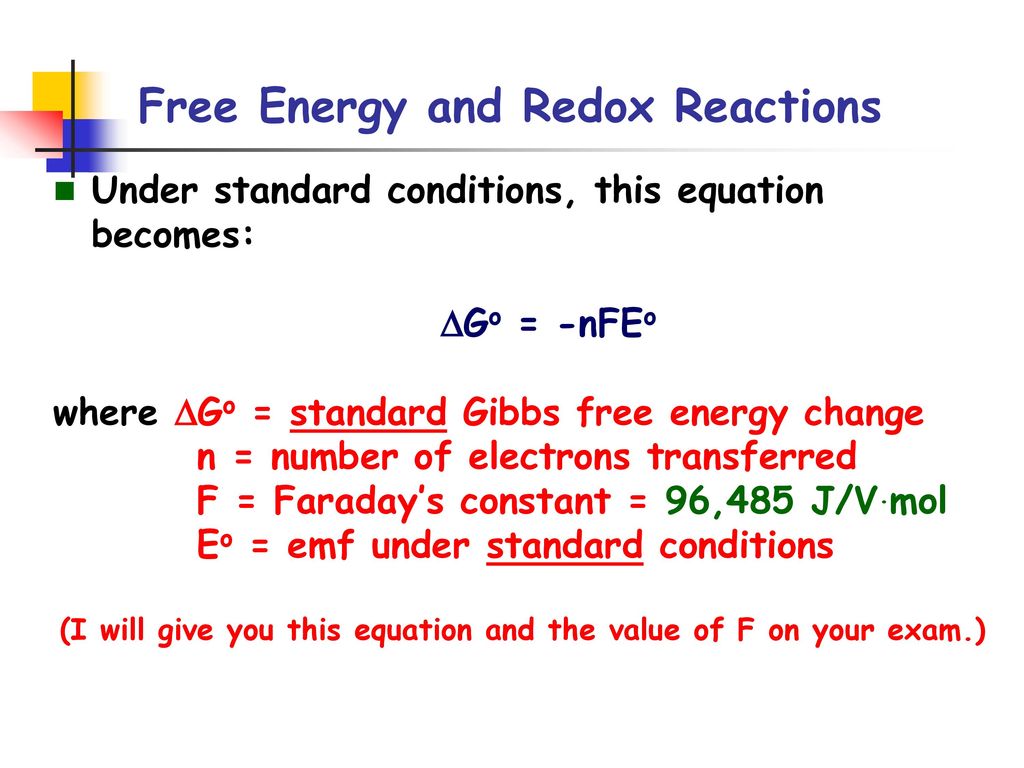
Free Energy And Redox Reactions Ppt Download

17 1 Equilibrium And Gibbs Free Energy Hl Youtube

High Temperature Muteriulr And Aonuses Standard Gibbs Free Energies Of Download Table

Cell Potential And Free Energy Protocol

Gibbs Free Energy Change Gibbs Equation Cie A Level Chemistry Revision Notes
Post a Comment for "Gibbs Free Energy Calculator Electrochemistry"