Non Standard Gibbs Free Energy Calculator
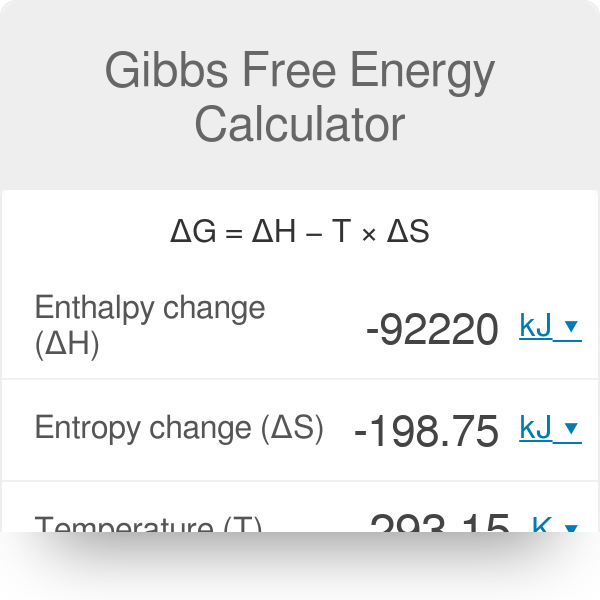
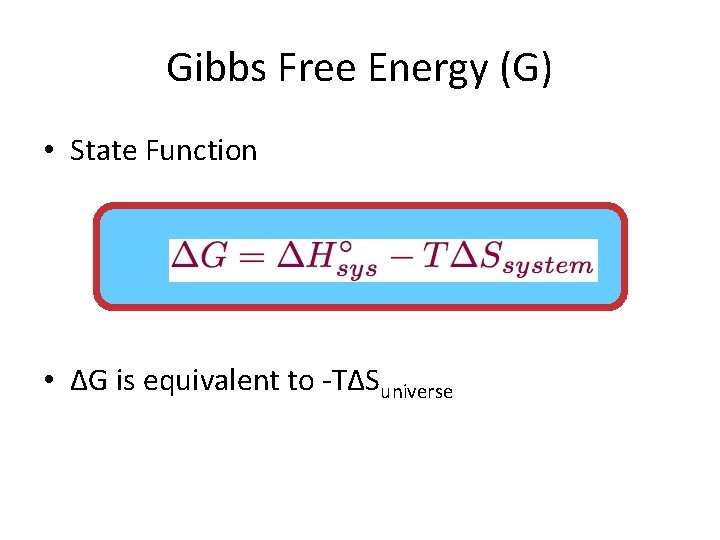
G o for a reaction can be calculated from tabulated standard-state free energy data. Standard Gibbs free energy of formation of a compound can be calculated using standard enthalpy of formation H absolute standard entropy S and standard temperature T 29815 K.

Standard Gibbs Free Energy Changes G For Anaerobic Reactions Ph 7 Download Table
The entropy of a system at 3371 K increases by 2217 JmolK.
Non standard gibbs free energy calculator. Is non-spontaneous at 300 K and becomes spontaneous at higher temperatures. Gibbs free energy G usually has the units kilojoules per mole kJ mol -1 The change in Gibbs free energy G for a chemical reaction at constant temperature T and pressure can be calculated. H -6428 KJmol 9.
Using Standard Molar Entropies Gibbs Free Energy Concepts and Calculations Vant Hoff Equation Environment Fossil Fuels Alternative Fuels Biological Examples DNA Structural Transitions etc Electrochemistry Balancing Redox Reactions GalvanicVoltaic Cells Calculating Standard Cell. When using thermodynamic tables you have to be very careful to remember that the substances referred to are in their standard statesFor liquids this is the pure liquid for solutions this will be at 1 molL concentrations and for gases at 1 bar atmospheric pressure. Standard Gibbs free energy of formation is the change in Gibbs free energy when elements in their standard states combine to form a product also in its standard state.
Watch more of this topic at httpclutchprepco24RhyNm Download this PDF. Exercise 463 Gibbs free energy calculations Q463-01 For the reaction. Httpclutchprepco24RhlK3 GET MORE CLUTCH.
How to Calculate Gibbs Free Energy. Calculating Standard Reaction Entropies eg. 3NO 2 g H 2 O l.
3HC CHg C 6H 6g H - 5973 kJ and S - 033 kJ K-1. In other words if a closed system goes through a change or process that transforms energy. Calculating Gibbs Free Energy.
Ag 2 O s 2 HNO 3 aq 2 AgNO 3 s H 2 O l o G -7175 KJmol 8. A system at 7765 K undergoes a change in enthalpy of 5. Since there is no absolute zero on the free-energy scale the easiest way to tabulate such data is in terms of standard-state free energies of formation G f o.
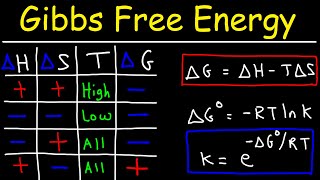
Calculate the standard change in Gibbs free energy kJ for the following reaction at 25 C. Calculating Standard Reaction Entropies eg. Gibbs Free Energy calculator uses gibbs_free_energy Enthalpy- TemperatureEntropy to calculate the Gibbs Free Energy Gibbs Free Energy is a thermodynamic potential that can be used to calculate the maximum of reversible work that may be performed by a thermodynamic system at a constant temperature and pressure.
Calculate the change in enthalpy of this system. Under normal conditions the pressure dependence of free energy is not important for solids and liquids because of their small molar volumes. 79 124 ratings Problem Details.
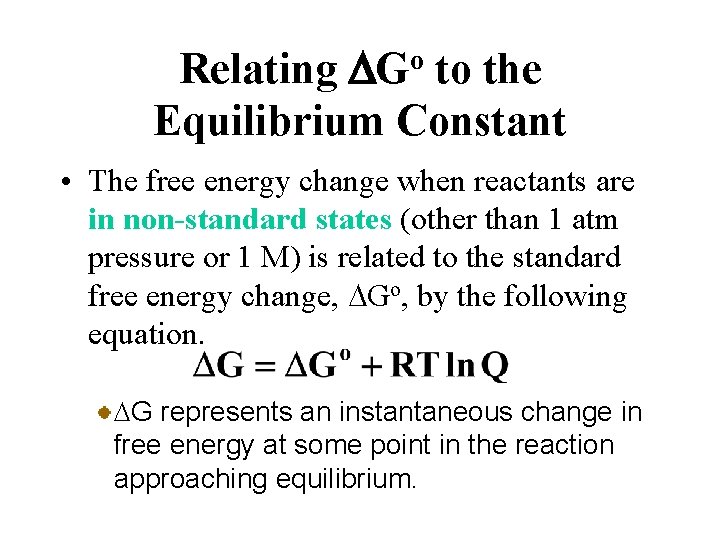
Gibbs free energy is a term used in physics specifically in thermodynamics that describes the maximum amount of reversible work that can be performed on a system. G G RT ln Q where R is the ideal gas constant 8314 Jmol K Q is the reaction quotient and T is the temperature in Kelvin. Standard-State Free Energies of Reaction.
For reactions that involve gases however the effect of pressure on free energy is very important. The free energy value is found to be 7175 kJmol. 378 Zeilen The term standard state is used to describe a reference state for substances and is a.
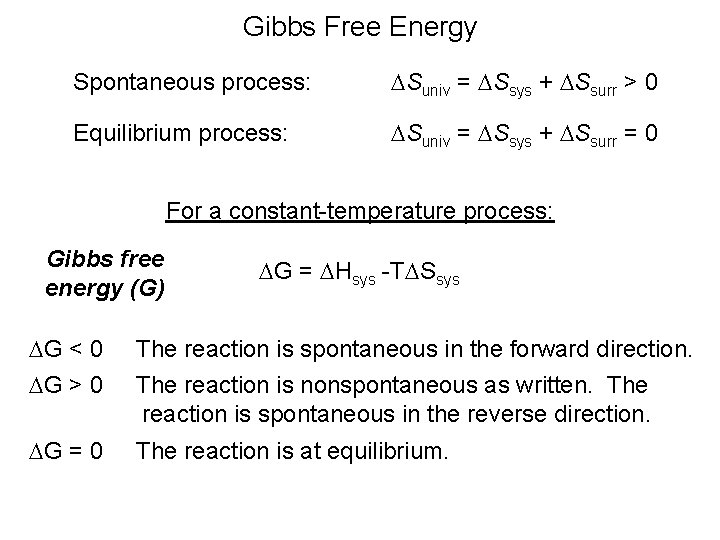
Calculate the standard Gibbs Free Energy for the following reaction. Is spontaneous at 300 K and becomes non-spontaneous at lower temperatures. This system must be at a constant temperature and pressure.
C 2010 Mark Rosengarten. Is spontaneous at 300 K and becomes non-spontaneous at higher temperatures. We are not given those values in the problem so we would have to search for it in literature.
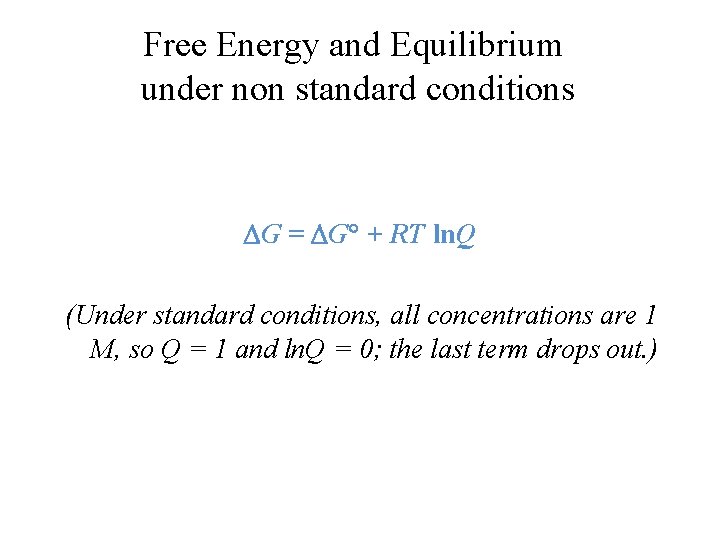
The change in Gibbs free energy under nonstandard conditions G can be determined from the standard change in Gibbs free energy G . 2nd Law of Themodynamics Entropy of Universe Gibbs free energy httpsyoutube1Wch4nK6ZMkStandard State Gibbs Free Energy vs NonStandard State Gibbs Free. Using Standard Molar Entropies Gibbs Free Energy Concepts and Calculations Vant Hoff Equation Environment Fossil Fuels Alternative Fuels Biological Examples DNA Structural Transitions etc Electrochemistry Balancing Redox Reactions GalvanicVoltaic Cells Calculating Standard Cell.
To calculate the Gibbs free energy of reaction we need to use the Gibbs free energy of formation for each of the compounds present in the reaction. As might be expected the standard-state free energy of. Gibbs Free Energy Definition.
In this last video we go over the equation for non-standard conditions and we use to derive the thermodynamic equation that explains Le Chaterliers Principle. VISIT our website for more of the. G V P.

Standard Molar Gibbs Free Energy For Reductive Processes Download Table

Gibbs Free Energy How Can We Use H

The Gibbs Free Energy Results From The Sr Method For The Empty Hydrate Download Table

Ppt Ch 27 More Gibbs Free Energy Powerpoint Presentation Free Download Id 3199497
Gibbs Free Energy How Can We Use H

Chapter 17 Free Energy And Thermodynamics 17 2
Entropy Free Energy And Equilibrium Chapter 18 Thermodynamics
Entropy Free Energy And Equilibrium Chapter 18 Thermodynamics
Gibbs Free Energy Entropy Enthalpy Equilibrium Constant K Youtube

The Gibbs Free Energy Difference Between And Phases Of Pure Iron Is Download Scientific Diagram

Getting Gibbs Energy As A Function Of Temperature

The Gibbs Free Energy Changes And The Steady State Fluxes The Download Scientific Diagram
Gibbs Free Energy Equilibrium Thermodynamics

Gibbs Free Energy Of Reaction Spreadsheet Youtube

Gibbs Free Energy Will It Happen Spontaneously Free Energy Science Blog Molecule Tattoo

Extrapolation Of The Calculated Free Energy Values At Standard Stage Download Table

Post a Comment for "Non Standard Gibbs Free Energy Calculator"